Relationship Between Concentration And Absorbance
The Beer-Lambert Law (also called Beer'south Constabulary) is a relationship between the attenuation of light through a substance and the properties of that substance. In this article, the definitions of transmittance and absorbance of light past a substance are get-go introduced followed by an explanation of the Beer-Lambert Constabulary.
What are transmittance and absorbance?
Consider monochromatic light transmitted through a solution; with an incident intensity of I0 and a transmitted intensity of I (Figure 1).
The transmittance, T, of the solution is defined equally the ratio of the transmitted intensity, I , over the incident intensity, I0 and takes values betwixt 0 and 1.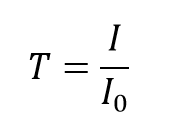 However, information technology is more unremarkably expressed every bit a percentage transmittance:
However, information technology is more unremarkably expressed every bit a percentage transmittance: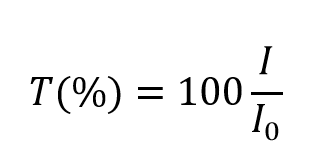 The absorbance, A, of the solution is related to the transmittance and incident and transmitted intensities through the following relations:
The absorbance, A, of the solution is related to the transmittance and incident and transmitted intensities through the following relations:
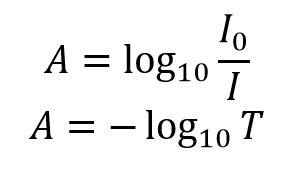 The absorbance has a logarithmic human relationship to the transmittance; with an absorbance of 0 corresponding to a transmittance of 100% and an absorbance of 1 corresponding to 10% transmittance. Additional values of transmittance and absorbance pairings are given in Table 1. A visual demonstration of the effect that the absorbance of a solution has on the attenuation light passing through information technology is shown Figure 2, where a 510 nm laser is passed through three solutions of Rhodamine 6G with different absorbance.
The absorbance has a logarithmic human relationship to the transmittance; with an absorbance of 0 corresponding to a transmittance of 100% and an absorbance of 1 corresponding to 10% transmittance. Additional values of transmittance and absorbance pairings are given in Table 1. A visual demonstration of the effect that the absorbance of a solution has on the attenuation light passing through information technology is shown Figure 2, where a 510 nm laser is passed through three solutions of Rhodamine 6G with different absorbance.
Table one: Absorbance and Transmittance Values:
| Absorbance | Transmittance |
|---|---|
| 0 | 100% |
| 1 | x% |
| 2 | 1% |
| iii | 0.1% |
| 4 | 0.01% |
| 5 | 0.001% |
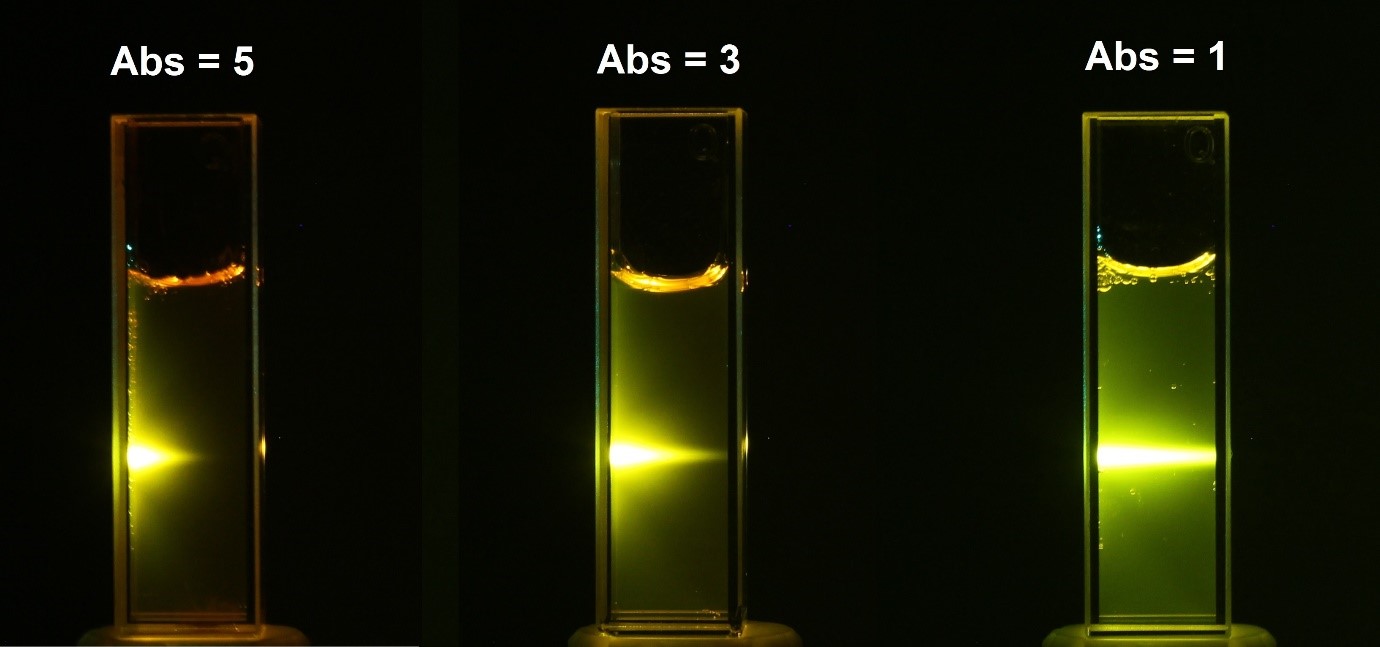
Effigy ii: Attenuation of a 510 nm laser through three solutions of Rhodamine 6G with different absorbance values at 510 nm. The yellow glow is the fluorescence emission at ~560 nm.
Absorbance is a dimensionless quantity and should, therefore, be unitless. Yet, information technology is quite common to see units of AU stated after the absorbance which are to said to either represent arbitrary units or absorbance units. These units are redundant and should be avoided. Another common come across is the use of the term optical density or OD in place of absorbance. Optical density is an older term that, in the context of assimilation spectroscopy, is synonymous with absorbance; still, the use of optical density in place of absorbance is discouraged by the IUPAC.ane
What is the Beer-Lambert Law?
The Beer-Lambert police is a linear human relationship between the absorbance and the concentration, molar absorption coefficient and optical coefficient of a solution: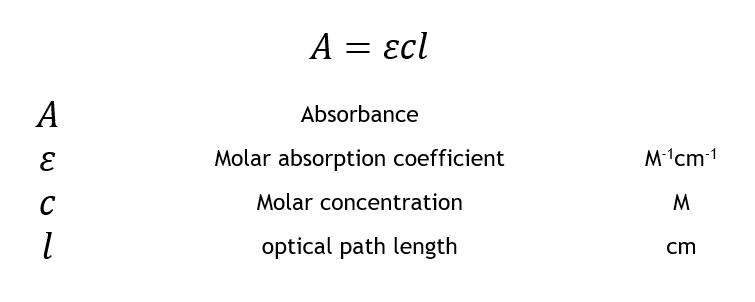
The tooth absorption coefficient is a sample dependent property and is a measure of how stiff an absorber the sample is at a item wavelength of light. The concentration is simply the moles L-ane (M) of the sample dissolved in the solution, and the length is the length of the cuvette used for the absorbance measurement and is typically 1 cm.
The Beer-Lambert police force states that there is a linear human relationship betwixt the concentration and the absorbance of the solution, which enables the concentration of a solution to be calculated past measuring its absorbance. To demonstrate this linear dependence five solutions of Rhodamine B in water were measured using the DS5 Dual Beam Spectrophotometer (Figure 3a) and from these absorption spectra, a linear scale curve of the absorbance versus concentration was created (Effigy 3b). Using this calibration bend the concentration of an unknown Rhodamine B solution can be adamant by measuring its absorbance which is the chief utility of the Beer-Lambert Constabulary.
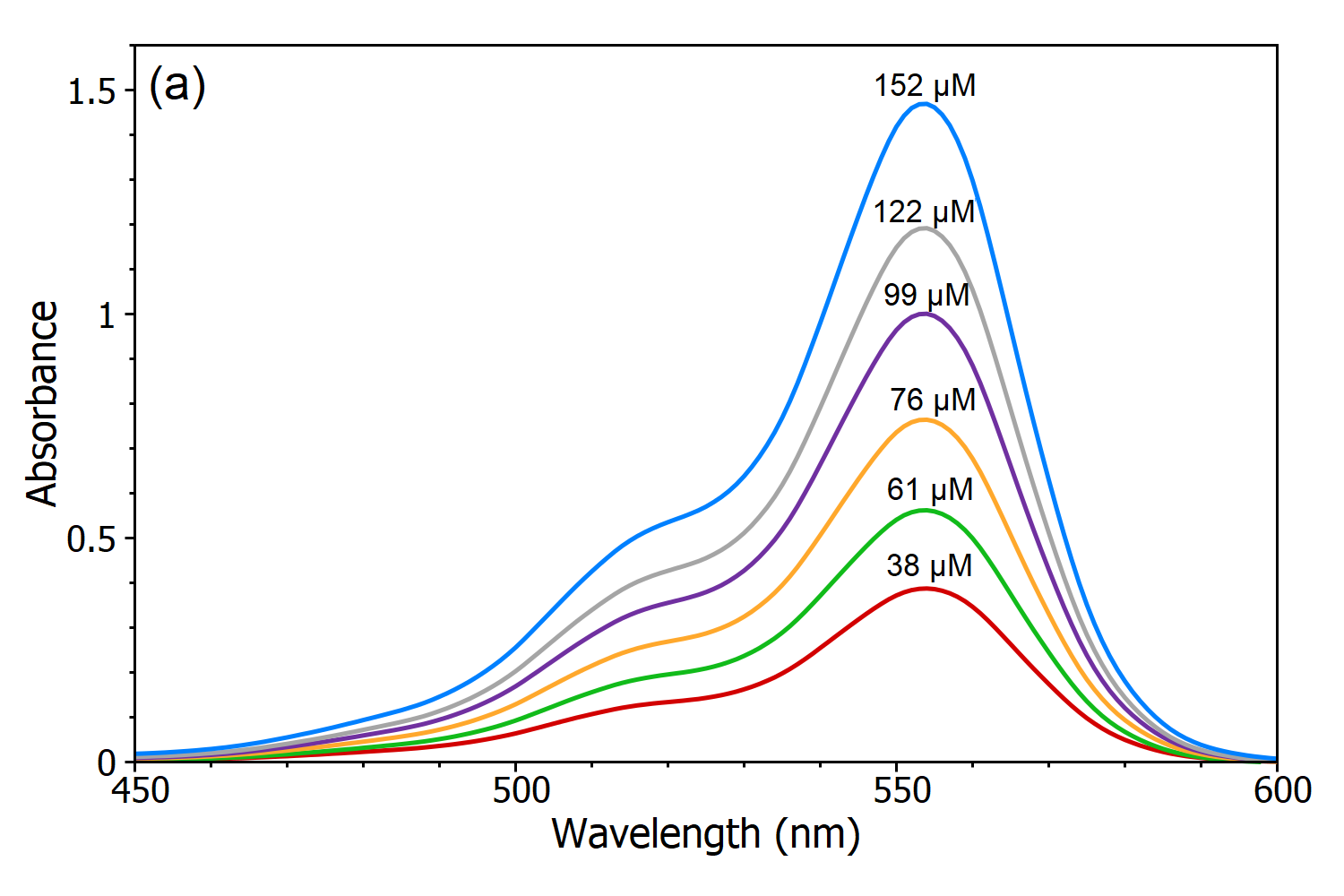

Figure 3 (a): Absorption spectra of Rhodamine B solutions with different concentrations in water measured using the DS5 Dual Beam Spectrophotometer. (b) Calibration curve of Rhodamine B in the h2o at measured at λmax.
For more than information on the theory of absorption spectroscopy, check out the frequently asked questions department on our blog.
References
one. IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gilt Book"); Compiled past A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications (1997)
Fluorescence Spectroscopy Equipment for Assimilation/Transmission Measurements
Edinburgh Instruments range of loftier end fluorescence spectroscopy equipment are perfect for absorption/transmission measurements. Why not browse our range below:
- FS5 Spectrofluorometer
- FLS chiliad Photoluminescence Spectrofluorometer
- DS5 Dual Beam UV-Vis Spectrophotometer
Proceed in Touch
If you have enjoyed reading our article on the Beer Lambert Law and would like to keep up to date with our latest articles, inquiry and product news, why not sign upwards to our monthly eNewsletter via the Sign-Up button beneath. Alternatively, join us on your favourite social media channel via the buttons at the bottom of the page.
Relationship Between Concentration And Absorbance,
Source: https://www.edinst.com/blog/the-beer-lambert-law/
Posted by: rochastemblitrand84.blogspot.com


0 Response to "Relationship Between Concentration And Absorbance"
Post a Comment